Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Hoshino, Tsuyoshi
Fusion Engineering and Design, 88(11), p.2956 - 2959, 2013/11
Times Cited Count:61 Percentile:97.76(Nuclear Science & Technology)The tritium as a fuel for fusion reactors is produced by the reaction of lithium-6 ( Li) with neutron in tritium breeding material. However, Li is one of 31 rare metal elements. Furthermore, as a means of addressing global warming, the world is increasingly turning to the use of Li-ion batteries in electric vehicles and as storage batteries in the home; therefore, there is a growing need for Li. In view of Japanese high dependence on imports for material resources, the securing of enough Li resources is an important policy for domestic industry in Japan. We proposed new method for Li recovery from seawater. The method involves the use of an ionic liquid through which only the Li ions in seawater and not the other ions, including Na, Mg, Ca and K, permeate from the anode side to the cathode side during electrodialysis. Thus, the Li ions become concentrated on the cathode side and can be recovered. With both ends of an ionic liquid covered with a SELEMION
Li) with neutron in tritium breeding material. However, Li is one of 31 rare metal elements. Furthermore, as a means of addressing global warming, the world is increasingly turning to the use of Li-ion batteries in electric vehicles and as storage batteries in the home; therefore, there is a growing need for Li. In view of Japanese high dependence on imports for material resources, the securing of enough Li resources is an important policy for domestic industry in Japan. We proposed new method for Li recovery from seawater. The method involves the use of an ionic liquid through which only the Li ions in seawater and not the other ions, including Na, Mg, Ca and K, permeate from the anode side to the cathode side during electrodialysis. Thus, the Li ions become concentrated on the cathode side and can be recovered. With both ends of an ionic liquid covered with a SELEMION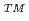 to prevent outflow of the ionic liquid, Li concentration increased from 4.5% after 2 h to 11.0% after 24 h with an applied electric voltage of 2 V.
to prevent outflow of the ionic liquid, Li concentration increased from 4.5% after 2 h to 11.0% after 24 h with an applied electric voltage of 2 V.
 at high temperatures up to 2190 K
at high temperatures up to 2190 KMorimoto, Kyoichi; Kato, Masato; Ogasawara, Masahiro*
Journal of Nuclear Materials, 443(1-3), p.286 - 290, 2013/11
Times Cited Count:5 Percentile:38.62(Materials Science, Multidisciplinary)In this study, measurement was conducted for the sliced MOX pellets containing 30% of Pu prepared by a conventional powder metallurgy technology. Oxygen-to-metal (O/M) ratios of the samples were adjusted in the range from 1.92 to 2.00. The thermal diffusivities of these samples were measured at temperature up to 2150 K with the laser flash method. Thermal diffusivities of the near-stoichiometric samples obtained in the cooling process were greatly lower than those in the heating process unlike measurement below 1770 K. On the other hand, they were almost identical for the sample of 1.946 in O/M. It was also shown that thermal diffusivity decreased with the temperature but increased with the O/M.
Takeuchi, Tomoaki; Kakubo, Yuta*; Matsukawa, Yoshitaka*; Nozawa, Yasuko*; Nagai, Yasuyoshi*; Nishiyama, Yutaka; Katsuyama, Jinya; Onizawa, Kunio; Suzuki, Masahide
Journal of Nuclear Materials, 443(1-3), p.266 - 273, 2013/11
Times Cited Count:16 Percentile:76.68(Materials Science, Multidisciplinary)Investigation on irradiation effects of weld-overlay claddings is necessary for safety assessment of reactor pressure vessels. We investigated microstructural changes in the cladding, which was composed of about 90% austenite and 10%  -ferrite phases, subjected to the neutron irradiation to 7.2
-ferrite phases, subjected to the neutron irradiation to 7.2 10
10 n/cm
n/cm at 290
at 290 C, by 3D atom probe tomography technique. In the ferrite phase, the amplitude of the Cr and Si concentration fluctuation was increased by the irradiation and Ni and Mn concentration fluctuations were newly occurred. In the austenite phase,
C, by 3D atom probe tomography technique. In the ferrite phase, the amplitude of the Cr and Si concentration fluctuation was increased by the irradiation and Ni and Mn concentration fluctuations were newly occurred. In the austenite phase,  '(Ni
'(Ni Si) -like clusters were formed. In contrast, the results of our previous work on the cladding subjected to thermal aging showed the amplitude of the Cr fluctuation was significantly increased and G (Ni-Si-Mn) phase was formed in the ferrite phase. Moreover, no changes were observed in the austenite by the aging.
Si) -like clusters were formed. In contrast, the results of our previous work on the cladding subjected to thermal aging showed the amplitude of the Cr fluctuation was significantly increased and G (Ni-Si-Mn) phase was formed in the ferrite phase. Moreover, no changes were observed in the austenite by the aging.
Otobe, Haruyoshi
Journal of Nuclear Materials, 442(1-3), p.394 - 399, 2013/11
Times Cited Count:1 Percentile:10.69(Materials Science, Multidisciplinary)The chemical properties (ex. atomic diffusion), the thermal properties (ex. melting point and thermal diffusivity) and the mechanical properties of oxide fuels are significantly dependent on the oxygen defects of the oxide fuels. For the first step to specify the oxygen defects of oxide fuels, we have tried the thermodynamic approaches to the oxygen defects by comparing the relations between the molar ratio of oxygen to metal (O/M) and the oxygen potentials of Pu, Am, Cm, Ce oxides and several solid solutions including Zr and Np dioxides. Consequently, we have found the consistent properties in the relations between O/M and the oxygen potentials of actinide, lanthanide oxides and their solid solutions, in addition to the dependence of the relations on the crystal structures.
 O
O (001) surface; A DFT study
(001) surface; A DFT studyMao, W.*; Chikada, Takumi*; Shimura, Kenichiro*; Suzuki, Akihiro*; Yamaguchi, Kenji; Terai, Takayuki*
Journal of Nuclear Materials, 443(1-3), p.555 - 561, 2013/11
Times Cited Count:3 Percentile:25.73(Materials Science, Multidisciplinary)In this work,  calculations based on density functional theory (DFT) and generalized gradient approximation were performed to investigate the structural and electronic properties of the cubic Er
calculations based on density functional theory (DFT) and generalized gradient approximation were performed to investigate the structural and electronic properties of the cubic Er O
O (001) surface and H adsorption processes on this surface. Several stable adsorption sites were identified, and at the most energetically favorable adsorption sites it was found that H bonds with O atoms at the cubic Er
(001) surface and H adsorption processes on this surface. Several stable adsorption sites were identified, and at the most energetically favorable adsorption sites it was found that H bonds with O atoms at the cubic Er O
O (001) surface with an adsorption energy of 295.68 kJ mol
(001) surface with an adsorption energy of 295.68 kJ mol at coverage 1/8 ML, which was inclined to decrease with the increase of H coverage (
at coverage 1/8 ML, which was inclined to decrease with the increase of H coverage ( 1/4 ML). In addition, the calculations revealed that the dissociative H atom configurations have adsorption energies that are at least 152.64 kJ mol
1/4 ML). In addition, the calculations revealed that the dissociative H atom configurations have adsorption energies that are at least 152.64 kJ mol greater than the H
greater than the H molecule configurations on the surface. These results are discussed in regard of the hydrogen isotope permeation behavior in the tritium permeation barrier in a fusion reactor.
molecule configurations on the surface. These results are discussed in regard of the hydrogen isotope permeation behavior in the tritium permeation barrier in a fusion reactor.
Amaya, Masaki; Nagase, Fumihisa
Journal of Nuclear Materials, 440(1-3), p.457 - 466, 2013/09
Times Cited Count:7 Percentile:49.28(Materials Science, Multidisciplinary)The relationship between oxidation amount and the activation energy of cladding oxidation by steam was investigated by thermogravimetry. Ring-shaped specimen which was prepared from a Zircaloy-4 cladding tube was hanged in a themobalance, and specimen weight gain due to oxidation was continuously measured in the temperature range from room temperature to  1400
1400 C under argon-steam gas flow. The activation energy of steam oxidation reaction was evaluated based on measured weight gains by applying a non-isothermal kinetics theory. It was found that the activation energy of oxidation depends on the amount of specimen weight gain. The activation energies showed constant values of
C under argon-steam gas flow. The activation energy of steam oxidation reaction was evaluated based on measured weight gains by applying a non-isothermal kinetics theory. It was found that the activation energy of oxidation depends on the amount of specimen weight gain. The activation energies showed constant values of  -300 and
-300 and  -180 kJ/mol in the range between 30 and 120 g/m
-180 kJ/mol in the range between 30 and 120 g/m and above 120 g/m
and above 120 g/m , respectively. The activation energy in the weight gain above 120 g/m
, respectively. The activation energy in the weight gain above 120 g/m agreed well with literature values. The specimen weight gains were formulated using obtained activation energies.
agreed well with literature values. The specimen weight gains were formulated using obtained activation energies.
Nakamichi, Masaru; Kim, Jae-Hwan
Journal of Nuclear Materials, 440(1-3), p.530 - 533, 2013/09
Times Cited Count:24 Percentile:87.01(Materials Science, Multidisciplinary)DEMO reactors require advanced neutron multiplier that has higher stability at high temperature. Beryllides such as Be Ti are the most promising materials. A plasma sintering method has been proposed as new technique for rod fabrication. It was clarified that the beryllide could be simultaneously synthesized and jointed by the plasma sintering method. Using this plasma-sintered beryllide rod, prototype pebble of Be-Ti beryllide was fabricated by a rotating electrode method. The prototype pebbles with 1 mm in average diameter were successfully fabricated. However, compositional structure was changed by re-melting. These Be and Be
Ti are the most promising materials. A plasma sintering method has been proposed as new technique for rod fabrication. It was clarified that the beryllide could be simultaneously synthesized and jointed by the plasma sintering method. Using this plasma-sintered beryllide rod, prototype pebble of Be-Ti beryllide was fabricated by a rotating electrode method. The prototype pebbles with 1 mm in average diameter were successfully fabricated. However, compositional structure was changed by re-melting. These Be and Be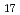 Ti
Ti phases in addition to Be
phases in addition to Be Ti phase were separated in the prototype pebble. From the result of annealing treatment of prototype pebble, prototype pebble phase was becoming single phase of Be
Ti phase were separated in the prototype pebble. From the result of annealing treatment of prototype pebble, prototype pebble phase was becoming single phase of Be Ti by annealing above 1473K.
Ti by annealing above 1473K.
Tanno, Takashi; Otsuka, Satoshi; Yano, Yasuhide; Kaito, Takeji; Oba, Yojiro*; Onuma, Masato*; Koyama, Shinichi; Tanaka, Kenya
Journal of Nuclear Materials, 440(1-3), p.568 - 574, 2013/09
Times Cited Count:17 Percentile:78.22(Materials Science, Multidisciplinary)This study carried out mechanical tests and microstructure characterizations of several 9Cr and 11Cr-ODS tempered martensitic steels, and discussed the appropriate chemical composition range of 11Cr-ODS tempered martensitic steel from the viewpoint of high-temperature strength improvement. It was shown that the residual  -ferrite fraction in 11Cr-ODS steel was successfully controlled to the same level as the 9Cr-ODS steel by selecting the matrix chemical compositions on the basis of the multi-component phase diagram. The tensile strength decreased with decreasing W content from 2.0 to 1.4 wt%. On the other hand, creep strength at 973 K did not degrade by the decreasing W content. Both tensile strength and creep strength increased with increasing population of the nano-sized oxide particles. Small angle X-ray scattering analysis revealed that titanium and excess oxygen contents were key parameters in order to improve the dispersion condition of nano-sized oxide particles.
-ferrite fraction in 11Cr-ODS steel was successfully controlled to the same level as the 9Cr-ODS steel by selecting the matrix chemical compositions on the basis of the multi-component phase diagram. The tensile strength decreased with decreasing W content from 2.0 to 1.4 wt%. On the other hand, creep strength at 973 K did not degrade by the decreasing W content. Both tensile strength and creep strength increased with increasing population of the nano-sized oxide particles. Small angle X-ray scattering analysis revealed that titanium and excess oxygen contents were key parameters in order to improve the dispersion condition of nano-sized oxide particles.
Hayashi, Hirokazu; Takano, Masahide; Kurata, Masaki; Minato, Kazuo
Journal of Nuclear Materials, 440(1-3), p.477 - 479, 2013/09
Times Cited Count:5 Percentile:38.62(Materials Science, Multidisciplinary)Neptunium trichloride of high purity was synthesized by the solid-state reaction of neptunium nitride, which was prepared from the oxide by the carbothermic reduction method, and cadmium chloride in a similar manner as reported for synthesis of AmCl . Lattice parameters of hexagonal NpCl
. Lattice parameters of hexagonal NpCl were determined from the X-ray diffraction pattern to be a = 0.7421
were determined from the X-ray diffraction pattern to be a = 0.7421  0.0006 nm and c = 0.4268
0.0006 nm and c = 0.4268  0.0003 nm, which fairly agree with the reported values (a = 0.742
0.0003 nm, which fairly agree with the reported values (a = 0.742  0.001 nm and c = 0.4281
0.001 nm and c = 0.4281  0.0005 nm). Melting temperature of NpCl
0.0005 nm). Melting temperature of NpCl was measured with about 1 mg of the sample which was hermetically encapsulated in a gold crucible using a differential thermal analyzer with heating and cooling rate of 10 K/min in an argon gas flow (50 mL/min). The melting temperature of NpCl
was measured with about 1 mg of the sample which was hermetically encapsulated in a gold crucible using a differential thermal analyzer with heating and cooling rate of 10 K/min in an argon gas flow (50 mL/min). The melting temperature of NpCl was determined to 1070
was determined to 1070  3 K, which is close to the recommended value 1075
3 K, which is close to the recommended value 1075 30 K, which was derived from the mean value of the melting temperature for UCl
30 K, which was derived from the mean value of the melting temperature for UCl (1115K) and that for PuCl
(1115K) and that for PuCl (1041 K).
(1041 K).
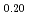 Pu
Pu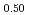 Am
Am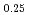 Cm
Cm )O
)O solid solutions
solid solutionsNishi, Tsuyoshi; Takano, Masahide; Akabori, Mitsuo; Arai, Yasuo
Journal of Nuclear Materials, 440(1-3), p.534 - 538, 2013/09
Times Cited Count:2 Percentile:18.63(Materials Science, Multidisciplinary)To clarify the dependence of thermal conductivity on storage time of curium containing oxide, the authors prepared the sintered sample of (Np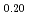 Pu
Pu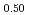 Am
Am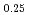 Cm
Cm )O
)O (x = 0.02, 0.04) solid solutions and evaluated the thermal conductivity. The thermal conductivities of (Np
(x = 0.02, 0.04) solid solutions and evaluated the thermal conductivity. The thermal conductivities of (Np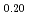 Pu
Pu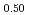 Am
Am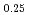 Cm
Cm )O
)O exponentially decreased with increasing storage duration. This result suggested that the degradation of the thermal conductivities was attributed to the accumulation of lattice defects by self-irradiation.
exponentially decreased with increasing storage duration. This result suggested that the degradation of the thermal conductivities was attributed to the accumulation of lattice defects by self-irradiation.
Takano, Masahide
Journal of Nuclear Materials, 440(1-3), p.489 - 494, 2013/09
Times Cited Count:8 Percentile:53.52(Materials Science, Multidisciplinary)The solid solution formation between ZrN and some lanthanide/transuranium (TRU) nitrides were examined by powder metallurgy of the nitride mixtures and simultaneous carbothermic nitridation of the oxide mixtures. Their solid solubility into ZrN matrix was determined by powder X-ray diffraction measurements as a function of relative lattice parameter difference (RLPD). The upper limit of RLPD value for the complete solid solubility is evaluated to be 8.6-8.9% in the temperature range of 1773-1973 K from the results of powder metallurgy. The solid solubility into ZrN decreases sharply at the greater RLPD value range. The solid solubility into ZrN in the products by carbothermic nitridation was lower, according to the influence of dissolved carbon impurity. The TRU composition limits for (Zr,TRU)N single-phase solid solution formation were simulated for the basis of fuel design works.
 simulated corium debris
simulated corium debrisTakano, Masahide; Nishi, Tsuyoshi
Journal of Nuclear Materials, 443(1-3), p.32 - 39, 2013/09
Times Cited Count:15 Percentile:72.14(Materials Science, Multidisciplinary)In order to clarify the possible impacts of sea salt deposit on the chemical and physical state of the fuel debris formed in the severe accident at Fukushima Daiichi Nuclear Power Plant, the high temperature reaction between sea salt deposit and (U,Zr)O simulated fuel debris (sim-debris) was examined in the temperature range from 1088 to 1668 K. The dense layer of calcium and/or sodium uranate formed on the surface of sim-debris pellet at 1275 K under airflow, with the thickness of over 50
simulated fuel debris (sim-debris) was examined in the temperature range from 1088 to 1668 K. The dense layer of calcium and/or sodium uranate formed on the surface of sim-debris pellet at 1275 K under airflow, with the thickness of over 50  m. When the oxygen partial pressure is low, calcium likely dissolve into the sim-debris to form solid solution. The diffusion depth was 5-6
m. When the oxygen partial pressure is low, calcium likely dissolve into the sim-debris to form solid solution. The diffusion depth was 5-6  m from the surface at 1275 K for 12 h. The crystalline MgO remains as the main residue stuck on the surface. A part of it can dissolve into the sim-debris depending on the temperature.
m from the surface at 1275 K for 12 h. The crystalline MgO remains as the main residue stuck on the surface. A part of it can dissolve into the sim-debris depending on the temperature.
Kim, Jae-Hwan; Nakamichi, Masaru
Journal of Nuclear Materials, 438(1-3), p.218 - 223, 2013/07
Times Cited Count:25 Percentile:87.76(Materials Science, Multidisciplinary) and zircaloy-2
and zircaloy-2Hirooka, Shun; Kato, Masato; Morimoto, Kyoichi; Komeno, Akira; Uchida, Teppei; Akashi, Masatoshi
Journal of Nuclear Materials, 437(1-3), p.130 - 134, 2013/06
Times Cited Count:5 Percentile:38.62(Materials Science, Multidisciplinary)Udagawa, Yutaka; Mihara, Takeshi; Fukuda, Takuji; Sugiyama, Tomoyuki; Suzuki, Motoe; Nagase, Fumihisa
no journal, ,
 system
systemNakayoshi, Akira; Kitawaki, Shinichi; Fukushima, Mineo; Murakami, Tsuyoshi*; Kurata, Masaki
no journal, ,
In the pyrochemical reprocessing of spent metallic fuels, electrorefining is conducted in a molten salt of LiCl-KCl eutectic (59:41 mol%) with dissolved actinide chlorides (AnCl ) at 773 K. Spent metallic fuels are dissolved in the molten salt at the anode, and actinide metals are deposited at the cathode. The concentration of AnCl
) at 773 K. Spent metallic fuels are dissolved in the molten salt at the anode, and actinide metals are deposited at the cathode. The concentration of AnCl in LiCl-KCl eutectic is assumed to be 2 mol%. However, the concentration of AnCl
in LiCl-KCl eutectic is assumed to be 2 mol%. However, the concentration of AnCl becomes higher in the vicinity of the anode during electrorefining, and the solubility of AnCl
becomes higher in the vicinity of the anode during electrorefining, and the solubility of AnCl may affect the rate of anodic dissolution. Therefore, it is important for the process operation to clarify the phase diagrams of LiCl-KCl-AnCl
may affect the rate of anodic dissolution. Therefore, it is important for the process operation to clarify the phase diagrams of LiCl-KCl-AnCl systems. In the present study, LiCl-KCl-UCl
systems. In the present study, LiCl-KCl-UCl ternary phase diagram in the range of 0
ternary phase diagram in the range of 0 20 mol% UCl
20 mol% UCl is investigated by a combination of X-ray diffraction method (XRD) and differential thermal analysis (DTA).
is investigated by a combination of X-ray diffraction method (XRD) and differential thermal analysis (DTA).

Matsunaga, Junji*; Kashibe, Shinji*; Serizawa, Hiroyuki; Oishi, Yuji*; Yamanaka, Shinsuke*
no journal, ,
We applied the helium infusion technique by a hot isothermal pressing (HIP) method and CeO was used for helium infusion. A sintered CeO
was used for helium infusion. A sintered CeO pellet was reduced in hydrogen atmosphere at 1073 K for 1 hour. O/Ce ratio of as-sintered CeO
pellet was reduced in hydrogen atmosphere at 1073 K for 1 hour. O/Ce ratio of as-sintered CeO and reduced CeO
and reduced CeO was evaluated by XRD as 2.00 and 1.99, respectively. The helium infusion was conducted under the experimental condition at 1473 K and 50 MPa of helium for CeO
was evaluated by XRD as 2.00 and 1.99, respectively. The helium infusion was conducted under the experimental condition at 1473 K and 50 MPa of helium for CeO and CeO
and CeO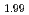 . As helium treated samples were annealed at 1773 K under atmospheric pressure in argon. By the heat treatment, additional intra- and intergranular bubbles were formed in CeO
. As helium treated samples were annealed at 1773 K under atmospheric pressure in argon. By the heat treatment, additional intra- and intergranular bubbles were formed in CeO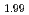 . However, such kind of bubble was not found in the stoichiometric CeO
. However, such kind of bubble was not found in the stoichiometric CeO . The sizes of intragranular bubbles in CeO
. The sizes of intragranular bubbles in CeO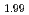 were about less than 100 nm, and intergranular bubbles were larger than those in grain. We at this consider that helium can dissolve into CeO
were about less than 100 nm, and intergranular bubbles were larger than those in grain. We at this consider that helium can dissolve into CeO matrix and oxygen vacancy would increase solubility of helium.
matrix and oxygen vacancy would increase solubility of helium.
Amamoto, Ippei; Kobayashi, Hidekazu; Yokozawa, Takuma; Yamashita, Teruo; Nagai, Takayuki; Suzuki, Yoshikazu*; Takebe, Hiromichi*; Mitamura, Naoki*; Tsuzuki, Tatsuya*
no journal, ,
The great amount of water used for cooling the stricken reactors at Fukushima Dai-ichi Nuclear Power Plant following the earthquake and tsunami of 11 March 2011 had resulted in accumulation of "remaining water" in some buildings. From public announcements, it seems likely the decontamination process of La Hague reprocessing plant would be employed as one of the treatment processes for the remaining water contaminated by FPs such as Cs, Sr, etc.. Based on literature study, Cs would precipitate with ferrocyanide compound as well as Sr with BaSO by applying the above-mentioned process. In this study, BaSO
by applying the above-mentioned process. In this study, BaSO as the simulated sludge was loaded into the iron phosphate glass (IPG) medium under different melting temperature. Based on the results, the performance of IPG containing BaO (decomposed from BaSO
as the simulated sludge was loaded into the iron phosphate glass (IPG) medium under different melting temperature. Based on the results, the performance of IPG containing BaO (decomposed from BaSO ) improved with increase loading of BaO up to 48 mol% at lower melting temperature.
) improved with increase loading of BaO up to 48 mol% at lower melting temperature.
Kurata, Yuji
no journal, ,
The compatibility of steels with lead-bismuth is one of critical issues to develop accelerator driven systems and lead-bismuth cooled reactors. It is expected that Si-enriched steels have good compatibility with lead-bismuth. Type 316SS with addition of 2.5 wt.% Si and Mod.9Cr-1Mo steel with addition of 1.5 wt.% Si were produced for the purpose of nuclear applications. In this presentation, the results of corrosion tests of these steels in liquid lead-bismuth are reported. Corrosion tests were conducted at 550 C in lead-bismuth containing 2.5
C in lead-bismuth containing 2.5 10
10 wt.% of oxygen and in lead-bismuth containing 4.4
wt.% of oxygen and in lead-bismuth containing 4.4 10
10 wt.% of oxygen. The additions of 2.5 wt.% Si to 316SS and 1.5 wt.% Si to T91 had the effect of reducing oxide film thickness in liquid lead-bismuth where oxygen concentrations were high. However, it was found that the formed oxide films didn't have enough protectiveness to prevent Ni dissolution and the penetration of Pb and Bi in liquid lead-bismuth containing 4.4
wt.% of oxygen. The additions of 2.5 wt.% Si to 316SS and 1.5 wt.% Si to T91 had the effect of reducing oxide film thickness in liquid lead-bismuth where oxygen concentrations were high. However, it was found that the formed oxide films didn't have enough protectiveness to prevent Ni dissolution and the penetration of Pb and Bi in liquid lead-bismuth containing 4.4 10
10 wt.% of oxygen.
wt.% of oxygen.
Shibata, Akira; Chimi, Yasuhiro; Hanawa, Satoshi; Omi, Masao; Tsuchiya, Kunihiko
no journal, ,
JMTR is planning to perform in-pile IASCC (Irradation assisted stress corrosion cracking) growth tests using the JMTR. The objectives of this experiments are to understand the difference between in-pile IASCC growth behavior and out-of-pile SCC growth behavior, and to confirm the effectiveness of mitigation by lowering electrochemical corrosion potential (ECP). For this experiments, 0.5T-CT specimen loaded up to  7 kN (corresponding to K
7 kN (corresponding to K  30 MPa
30 MPa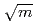 ), and the crack growth will be monitored by the Potential Drop Method in the irradiation capsule of the JMTR. Thus, the lever type loading unit was developed. ECP should be measured under irradiation condition in the reactor. The ECP sensor has been developed to determine the corrosive potential under high temperature and high pressure water condition. In this paper, development of the lever type loading unit and development of ECP sensor for in-pile IASCC growth tests are described.
), and the crack growth will be monitored by the Potential Drop Method in the irradiation capsule of the JMTR. Thus, the lever type loading unit was developed. ECP should be measured under irradiation condition in the reactor. The ECP sensor has been developed to determine the corrosive potential under high temperature and high pressure water condition. In this paper, development of the lever type loading unit and development of ECP sensor for in-pile IASCC growth tests are described.